Contaminated cough syrup deaths in Madhya Pradesh lead to the arrest of Sresan Pharma’s owner. Coldrif syrup found with toxic diethylene glycol. National crackdown on substandard drugs intensifies.
By Qalam Times News Network
Dateline: Chennai/Bhopal | October 9, 2025
Contaminated cough syrup has once again shaken India’s pharmaceutical industry after the deaths of 20 children in Madhya Pradesh were linked to Coldrif syrup, produced by Tamil Nadu-based Sresan Pharmaceuticals. The company’s owner, G. Ranganathan, was arrested from his Chennai apartment on Thursday by a seven-member team from the Madhya Pradesh Police.
Seventeen of the deaths occurred in Chhindwara district, while two were reported from Betul and one from Pandhurna. Deputy Chief Minister Rajendra Shukla confirmed that five other children are undergoing treatment for kidney failure.
Doctor’s Arrest Sparks Medical Backlash
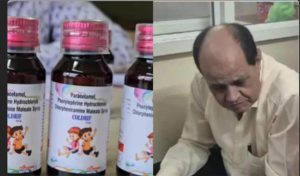
Before Ranganathan’s arrest, police had detained Dr. Praveen Soni, a government pediatrician from Chhindwara, who allegedly prescribed the contaminated syrup at his private clinic. His arrest drew strong criticism from the Indian Medical Association, which blamed systemic lapses in drug regulation rather than individual doctors.
Following the uproar, the Madhya Pradesh government filed a criminal case against Sresan Pharmaceuticals, whose Coldrif syrup samples were found to contain 48.6% diethylene glycol (DEG) — a toxic industrial chemical that can cause kidney failure and death even in small doses.
National and State-Level Crackdown
In response to the crisis, Punjab, Kerala, Tamil Nadu, Madhya Pradesh, Uttar Pradesh, and Jharkhand have all banned Coldrif syrup and launched investigations into Sresan Pharma. The Union Health Ministry has also issued an advisory warning against the prescription of cough and cold medicines to children below two years old, stressing that such drugs should only be used for older children under close medical supervision.
Meanwhile, in Rajasthan, three similar child deaths linked to another pharmaceutical company, Kayson Pharma, led to the suspension of a Drug Controller and the immediate halt of all 19 medicines from that firm.
Toxic Findings and Global Parallels
Laboratory tests confirmed that the contaminated Coldrif syrup contained diethylene glycol far beyond safe limits. The Tamil Nadu Directorate of Drug Control classified the syrup as “Not of Standard Quality,” prompting bans on both Coldrif and another syrup, Nextro-DS, pending further test results.
According to grieving families, the children initially suffered from mild colds and fevers, but their condition rapidly deteriorated after taking the syrup — leading to kidney failure and death. Post-mortem kidney biopsies revealed the presence of DEG contamination.
Government Response and Promises of Accountability
Madhya Pradesh Chief Minister Mohan Yadav called the deaths “extremely tragic” and vowed that “the guilty will not be spared.” He confirmed that a state-level team has been formed to investigate the incident, and that the Tamil Nadu government has been asked to cooperate in testing and verifying all products manufactured by Sresan Pharmaceuticals.
The Health Ministry said the samples “contained diethylene glycol beyond permissible limits,” and assured the public that strict measures would follow.
Since 2022, toxins found in Indian-made contaminated syrups have been linked to the deaths of at least 141 children in Gambia, Uzbekistan, and Cameroon — putting India’s drug safety system under renewed international scrutiny.







